Inquiry
Form loading...
Calcium iodate is a compound that combines calcium and iodine. It plays a significant role in various fields, such as nutrition and animal feed. This compound is vital for preventing iodine deficiency, which can lead to health issues.
In agriculture, calcium iodate serves as an essential source of iodine for livestock. Livestock that lack iodine may suffer from severe health problems. Including calcium iodate in animal diets helps ensure healthy growth and productivity.
Furthermore, calcium iodate is used in food fortification. It provides an accessible way to increase iodine levels in human diets. Many regions experience iodine deficiency, making this fortification critical. Despite its benefits, awareness about calcium iodate remains limited. More education on its importance is needed for better health outcomes.

Calcium Iodate is an inorganic compound with the formula Ca(IO3)2. It combines calcium and iodine in a stable form. This compound appears as white crystals or powders. It is highly soluble in water, making it easy to use in various applications. Many people encounter it in dietary supplements. It is primarily used to address iodine deficiency in populations.
The importance of calcium iodate lies in its nutritional value. Iodine is essential for thyroid hormone production. A lack of iodine can lead to health problems. These issues include goiter and developmental delays in children. Calcium iodate helps ensure adequate iodine intake, especially in regions with low dietary sources.
It’s not just a supplement. Calcium iodate plays a role in food fortification. However, it’s crucial to balance iodine intake. Too much can also be harmful. Some may overlook these details when considering supplements. Understanding the precise needs for iodine is vital for health.

Calcium iodate is an inorganic compound with the formula Ca(IO3)2. This compound is a source of iodine and calcium, which are vital for various biological processes. In many regions, iodine deficiency is a public health issue. Calcium iodate provides a solution by supplying both elements needed for health.
The chemical composition consists of calcium ions and iodate ions. Each molecule contains one calcium atom and two iodate groups. Iodate ions comprise one iodine atom bonded to three oxygen atoms. This simple yet effective combination yields a stable compound. The presence of iodine helps support thyroid function. Calcium plays a key role in bone health and muscle function.
Understanding calcium iodate's importance goes beyond chemistry. Many people do not realize its significance in nutrition. The use of calcium iodate in fortification helps combat iodine deficiency. Yet, the awareness regarding its benefits remains limited. How can we improve this? Education is crucial. Individuals should know the role of calcium iodate in maintaining proper health.
Calcium iodate is a chemical compound that plays a significant role in various industries. Its manufacturing process is quite fascinating. Typically, it involves the reaction of calcium hydroxide with iodine in an aqueous solution. The reaction is simple yet effective. Calcium hydroxide is soluble in water, making it easier to incorporate iodine. This step is critical for obtaining a proper yield.
The crystallization process follows the initial reaction. During crystallization, the solution cools, allowing calcium iodate to form solid crystals. These crystals can be collected and purified. Additionally, controlling temperature and pH levels is essential during this phase. Small deviations can lead to impurities, affecting the final product's quality.
In some cases, manufacturers face challenges like inconsistent iodine levels or impurities. These problems can hinder production efficiency. It is vital for manufacturers to monitor conditions closely. Minor adjustments can make a big difference in the product's quality. Continuous improvement in the manufacturing process is necessary for achieving optimal results.
| Property | Value |
|---|---|
| Chemical Formula | Ca(IO3)2 |
| Appearance | White crystalline powder |
| Solubility in Water | Slightly soluble |
| Molar Mass | 293.89 g/mol |
| Applications | Animal feed, food fortification, water treatment |
| Health Benefits | Prevent iodine deficiency, support thyroid function |
| Manufacturing Process | Reacting calcium carbonate with iodic acid |
| Safety Information | Generally recognized as safe in regulated amounts |
Calcium iodate is an important compound used in agriculture and nutrition. Its primary application lies in enhancing animal feed. Livestock often require iodine for proper growth and metabolism. Calcium iodate serves as a reliable iodine source, promoting healthier animals. This, in turn, can lead to higher milk production and improved overall animal health.
In crop production, calcium iodate is equally significant. It can be added to fertilizers. This ensures that plants receive adequate iodine, an essential nutrient. Iodine deficiency in soil can limit crop yield and quality. By using calcium iodate, farmers can address this issue effectively. As a result, crops are not only more productive but also more nutritious for consumers.
Controversy does exist regarding iodine supplementation. Some argue about proper dosage levels. Too much iodine can be harmful, leading to thyroid issues in animals and humans. It is crucial for farmers and nutritionists to monitor iodine levels in feed and soil. Striking a balance is essential to maximize benefits while minimizing risks. Attention to detail in application can prevent potential pitfalls in both agriculture and nutrition.
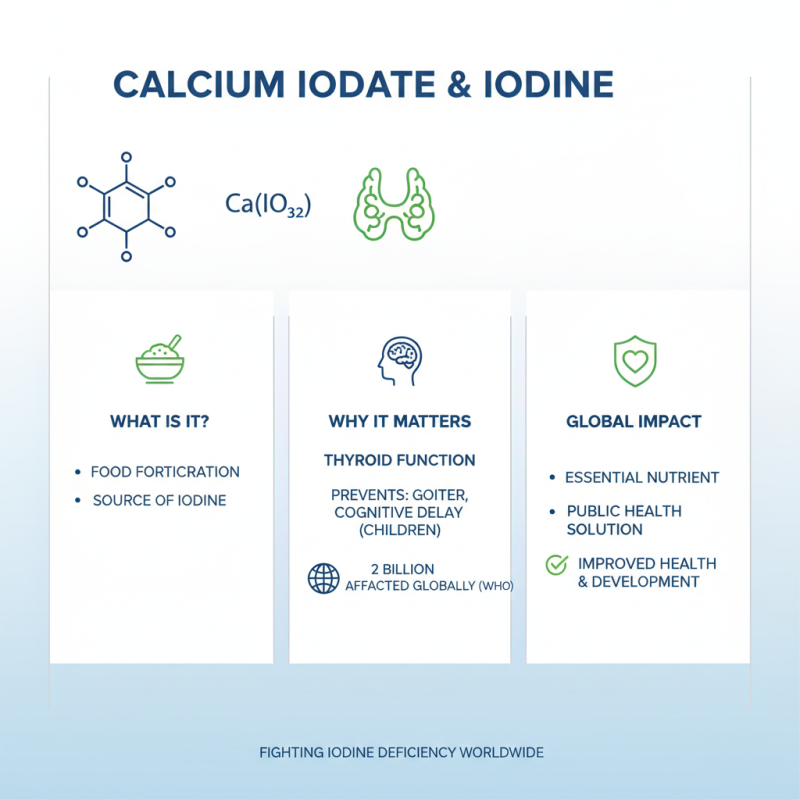
Calcium iodate is a compound that is often used in food fortification. It serves as a significant source of iodine, an essential nutrient necessary for thyroid function. According to the World Health Organization, iodine deficiency affects about 2 billion people globally. This deficiency can lead to severe health issues, including goiter and developmental delays in children.
Incorporating calcium iodate into diets can effectively reduce these health risks. Studies show that iodized salt or fortified foods containing calcium iodate can significantly enhance iodine levels in populations at risk. For instance, a report by the International Council for the Control of Iodine Deficiency Disorders suggests that daily intake of fortified foods can boost iodine levels by 20-50%. This improvement is vital for cognitive function and overall health.
While calcium iodate plays a crucial role, awareness of its importance is often lacking. Many people do not realize the impact of iodine on their health. Continued education about this compound is necessary. As we evaluate dietary needs, we must consider both the benefits of calcium iodate and the potential gaps in public awareness. Recognizing these shortcomings can guide better health strategies moving forward.